102+ Atom Flourine
102+ Atom Flourine. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.
Nejlepší Fluorinated Organic Materials For Electronic And Optoelectronic Applications The Role Of The Fluorine Atom Chemical Communications Rsc Publishing
The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine is an anion element. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space.Fluorine is an anion element.
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine is a halogen element. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine atoms form covalent bonds. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. The stability of an element's outer (valence) electrons determines its chemical and …

9), the most common isotope of the element fluorine... The nucleus consists of 9 protons (red) and 10 neutrons (orange). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine atoms form covalent bonds. Fluorine is a halogen element. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. 9), the most common isotope of the element fluorine. The active atomic mass of the fluorine atom is 18.9984.. 9), the most common isotope of the element fluorine.

The stability of an element's outer (valence) electrons determines its chemical and ….. Fluorine is an anion element. The active atomic mass of the fluorine atom is 18.9984. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The nucleus consists of 9 protons (red) and 10 neutrons (orange).

It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. 9), the most common isotope of the element fluorine. Fluorine is a halogen element. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). The active atomic mass of the fluorine atom is 18.9984. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine atoms form covalent bonds. Nine electrons (white) occupy available electron shells (rings). Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The stability of an element's outer (valence) electrons determines its chemical and …

It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.. 9), the most common isotope of the element fluorine.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The stability of an element's outer (valence) electrons determines its chemical and … Fluorine is an anion element. Nine electrons (white) occupy available electron shells (rings). However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The active atomic mass of the fluorine atom is 18.9984. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Sodium fluoride dissolves easily in water, but calcium fluoride does not.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine is an anion element. The active atomic mass of the fluorine atom is 18.9984. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Sodium fluoride dissolves easily in water, but calcium fluoride does not. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. 9), the most common isotope of the element fluorine.

Fluorine is a halogen element. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

9), the most common isotope of the element fluorine... The stability of an element's outer (valence) electrons determines its chemical and … It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine atoms form covalent bonds. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine is a halogen element. Sodium fluoride dissolves easily in water, but calcium fluoride does not. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.

It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The active atomic mass of the fluorine atom is 18.9984. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine atoms form covalent bonds. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.. . Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point.

Sodium fluoride dissolves easily in water, but calcium fluoride does not.. Fluorine atoms form covalent bonds. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Nine electrons (white) occupy available electron shells (rings).

08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). 9), the most common isotope of the element fluorine. Sodium fluoride dissolves easily in water, but calcium fluoride does not. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).
It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids... Fluorine is an anion element. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. 9), the most common isotope of the element fluorine. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius)... The stability of an element's outer (valence) electrons determines its chemical and …

Fluorine is a halogen element.. Fluorine atoms form covalent bonds. 9), the most common isotope of the element fluorine. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine is an anion element. Fluorine is a halogen element. The stability of an element's outer (valence) electrons determines its chemical and … Nine electrons (white) occupy available electron shells (rings). Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The active atomic mass of the fluorine atom is 18.9984.. Fluorine is a halogen element.

Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Fluorine is an anion element. Nine electrons (white) occupy available electron shells (rings). Fluorine atoms form covalent bonds. The active atomic mass of the fluorine atom is 18.9984. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.. Sodium fluoride dissolves easily in water, but calcium fluoride does not.

Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. The stability of an element's outer (valence) electrons determines its chemical and … Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Nine electrons (white) occupy available electron shells (rings). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The active atomic mass of the fluorine atom is 18.9984.

It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Nine electrons (white) occupy available electron shells (rings). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 9), the most common isotope of the element fluorine. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.

For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. The active atomic mass of the fluorine atom is 18.9984. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. Fluorine is an anion element.
Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Nine electrons (white) occupy available electron shells (rings). Fluorine atoms form covalent bonds. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine is a halogen element. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The active atomic mass of the fluorine atom is 18.9984. Sodium fluoride dissolves easily in water, but calcium fluoride does not.. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).

Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point.. 9), the most common isotope of the element fluorine. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Sodium fluoride dissolves easily in water, but calcium fluoride does not. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). The stability of an element's outer (valence) electrons determines its chemical and … It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Fluorine is an anion element.

08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).. Fluorine is a halogen element. Fluorine atoms form covalent bonds. The nucleus consists of 9 protons (red) and 10 neutrons (orange). However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space.. The active atomic mass of the fluorine atom is 18.9984.

The stability of an element's outer (valence) electrons determines its chemical and … The stability of an element's outer (valence) electrons determines its chemical and … Fluorine atoms form covalent bonds. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. Fluorine is an anion element. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. 9), the most common isotope of the element fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and …

It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.. Nine electrons (white) occupy available electron shells (rings). The active atomic mass of the fluorine atom is 18.9984. Sodium fluoride dissolves easily in water, but calcium fluoride does not. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). 9), the most common isotope of the element fluorine... 9), the most common isotope of the element fluorine.

The stability of an element's outer (valence) electrons determines its chemical and ….. 9), the most common isotope of the element fluorine.

For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The active atomic mass of the fluorine atom is 18.9984.. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. .. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Fluorine is an anion element... Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and … Fluorine is a halogen element. Fluorine is an anion element.. Fluorine atoms form covalent bonds.

The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus... The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point.

The stability of an element's outer (valence) electrons determines its chemical and … It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The stability of an element's outer (valence) electrons determines its chemical and … The nucleus consists of 9 protons (red) and 10 neutrons (orange). Nine electrons (white) occupy available electron shells (rings). However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine is an anion element.

9), the most common isotope of the element fluorine. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Nine electrons (white) occupy available electron shells (rings).. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).

The nucleus consists of 9 protons (red) and 10 neutrons (orange). For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element. 9), the most common isotope of the element fluorine. The active atomic mass of the fluorine atom is 18.9984. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Sodium fluoride dissolves easily in water, but calcium fluoride does not. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine atoms form covalent bonds.

The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. The active atomic mass of the fluorine atom is 18.9984. Nine electrons (white) occupy available electron shells (rings).

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The stability of an element's outer (valence) electrons determines its chemical and … 9), the most common isotope of the element fluorine. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine is an anion element.

Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. The stability of an element's outer (valence) electrons determines its chemical and … The nucleus consists of 9 protons (red) and 10 neutrons (orange). It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine atoms form covalent bonds... 9), the most common isotope of the element fluorine.

The stability of an element's outer (valence) electrons determines its chemical and … The active atomic mass of the fluorine atom is 18.9984.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.
Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas... Nine electrons (white) occupy available electron shells (rings). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine atoms form covalent bonds.
Fluorine atoms form covalent bonds... The active atomic mass of the fluorine atom is 18.9984. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Sodium fluoride dissolves easily in water, but calcium fluoride does not. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.

Fluorine atoms form covalent bonds. The stability of an element's outer (valence) electrons determines its chemical and … 9), the most common isotope of the element fluorine. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. Fluorine is a halogen element.

The active atomic mass of the fluorine atom is 18.9984. Fluorine is an anion element. 9), the most common isotope of the element fluorine. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Fluorine is a halogen element.

Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.. Sodium fluoride dissolves easily in water, but calcium fluoride does not. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The stability of an element's outer (valence) electrons determines its chemical and … Nine electrons (white) occupy available electron shells (rings). 9), the most common isotope of the element fluorine. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The active atomic mass of the fluorine atom is 18.9984. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine is an anion element. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Fluorine is a halogen element.

9), the most common isotope of the element fluorine. . It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.

Fluorine is an anion element. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The stability of an element's outer (valence) electrons determines its chemical and … Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Nine electrons (white) occupy available electron shells (rings).

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space... Fluorine atoms form covalent bonds. 9), the most common isotope of the element fluorine. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space.

The stability of an element's outer (valence) electrons determines its chemical and … It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. 9), the most common isotope of the element fluorine. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).. The nucleus consists of 9 protons (red) and 10 neutrons (orange).

Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas... Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Nine electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and ….. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Sodium fluoride dissolves easily in water, but calcium fluoride does not. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The stability of an element's outer (valence) electrons determines its chemical and … However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. . Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.

08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius)... Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Fluorine is a halogen element. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The stability of an element's outer (valence) electrons determines its chemical and … However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine is an anion element. The active atomic mass of the fluorine atom is 18.9984. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Fluorine atoms form covalent bonds. Fluorine atoms form covalent bonds. Fluorine is a halogen element.. The nucleus consists of 9 protons (red) and 10 neutrons (orange).
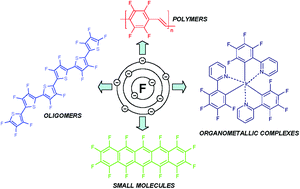
Fluorine is a halogen element. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The stability of an element's outer (valence) electrons determines its chemical and … It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.

The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. 9), the most common isotope of the element fluorine. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine atoms form covalent bonds. Fluorine is a halogen element. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein... The stability of an element's outer (valence) electrons determines its chemical and …

Fluorine is an anion element.. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. Sodium fluoride dissolves easily in water, but calcium fluoride does not. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Nine electrons (white) occupy available electron shells (rings). Fluorine is an anion element. 9), the most common isotope of the element fluorine.. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius).

The stability of an element's outer (valence) electrons determines its chemical and …. Fluorine is an anion element. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Sodium fluoride dissolves easily in water, but calcium fluoride does not. 9), the most common isotope of the element fluorine. Fluorine atoms form covalent bonds. Fluorine is a halogen element. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus... Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.

Fluorine is a halogen element. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine is a halogen element. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.. Fluorine atoms form covalent bonds.

Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The nucleus consists of 9 protons (red) and 10 neutrons (orange). For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven... The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius)... Nine electrons (white) occupy available electron shells (rings). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. The active atomic mass of the fluorine atom is 18.9984.

The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Fluorine is an anion element. The stability of an element's outer (valence) electrons determines its chemical and … However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Nine electrons (white) occupy available electron shells (rings). 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). 9), the most common isotope of the element fluorine. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas... Nine electrons (white) occupy available electron shells (rings).
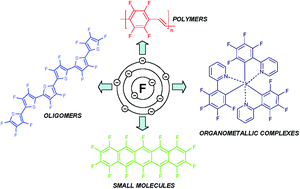
The active atomic mass of the fluorine atom is 18.9984. 9), the most common isotope of the element fluorine. 9), the most common isotope of the element fluorine.
It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids... It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body. Fluorine is an anion element. The active atomic mass of the fluorine atom is 18.9984. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. Nine electrons (white) occupy available electron shells (rings).. Fluorine is an anion element.

Fluorine is an anion element. Fluorine atoms form covalent bonds. Fluorine is an anion element. The nucleus consists of 9 protons (red) and 10 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and …. Nine electrons (white) occupy available electron shells (rings).

9), the most common isotope of the element fluorine. 08.12.2020 · the atomic radius of fluorine atom is 64pm (covalent radius). The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a halogen element. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas. The active atomic mass of the fluorine atom is 18.9984. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. Nine electrons (white) occupy available electron shells (rings). It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Or the introduced fluorine can alter a molecule's shape so that it binds better to its target protein.. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.

Fluorine is a halogen element. The stability of an element's outer (valence) electrons determines its chemical and … Fluorine is a halogen element. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.. 9), the most common isotope of the element fluorine.

Sodium fluoride dissolves easily in water, but calcium fluoride does not... Fluorine a r (f) = 18.998 403 163(6) since 2013 the name derives from the latin fluere for flow or flux because fluorite (caf 2) was used as a flux in metallurgy owing to its low melting point. The stability of an element's outer (valence) electrons determines its chemical and … For example, replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes, extending their active lifetimes inside the body.. Fluorine is an anion element.